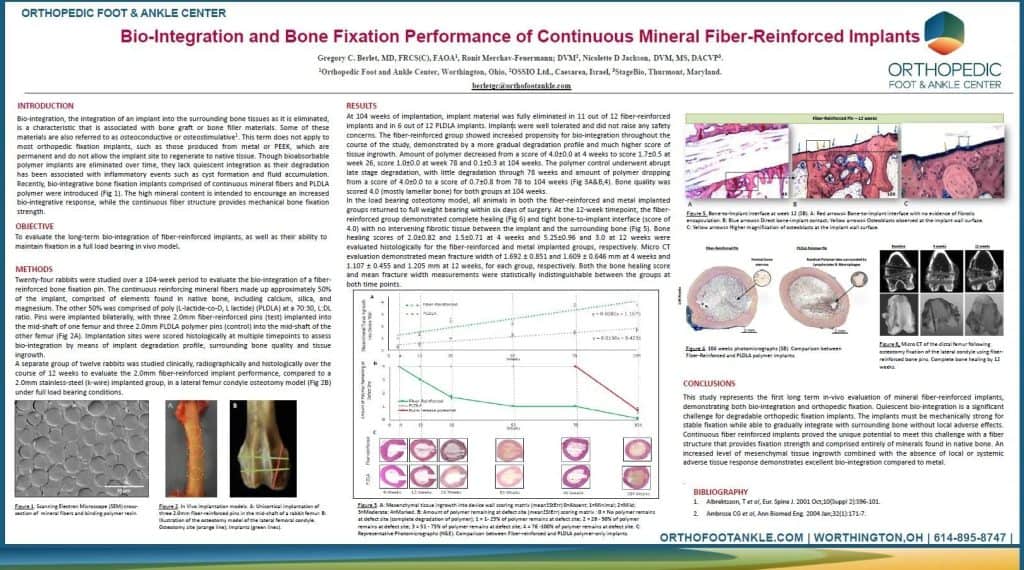
During the virtual 2020 AOFAS meeting, Greg Berlet, MD, presented a scientific ePoster on the bio-integration and bone fixation strength of OSSIOfiber Intelligent Bone Regeneration Technology. We invite you to read the ePoster!
AOFAS Scientific ePoster
Bio-Integration and Bone Fixation Performance of Continuous Mineral Fiber-Reinforced Implants
Authors:
Gregory Berlet, MD, FRCS(C), FAOA
Ronit Merchav-Feuermann, DVM
Nicolette D Jackson, DVM, MS, DACVP