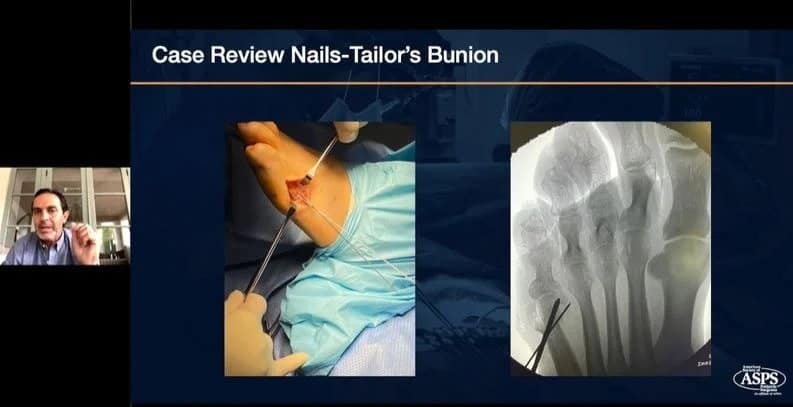
|
Earn CECH credits by watching this recent webinar, hosted with American Society of Podiatric Surgeons and presenter Bob Baravarian, DPM, FACFAS. NOW ON-DEMANDWEBINAR: The Science and Clinical Experience with Strong and Bio-Integrative Implant Fixation Speaker: Bob Baravarian, DPM, FACFAS
Hear Dr. Baravarian present on:
Log in to the ASPS website to access the webinar and earn CECH credit! Please note to receive CECH credit, active ASPS membership or additional access may be required. |
ASPS Webinar
Related Posts
Case Study: A Clinical Approach to Treating Hammertoe Deformities with a Bio-Integrative Fixation Implant
Hammertoe deformities are one of the most common foot deformities, estimated to affect one third of the population.1 Intramedullary implants have gained popularity over the past decade, with the advantage of avoiding an external, percutanous K-wire during the rehabilitation phase. There are many on the market, manufactured from a variety of implant materials including conventionalContinue reading “Case Study: A Clinical Approach to Treating Hammertoe Deformities with a Bio-Integrative Fixation Implant”
First-in-Human, Prospective, Multicenter Clinical Study
OSSIO is thrilled to announce the publication of our prospective, multicenter, first-in-human clinical trial in Foot & Ankle Orthopaedics. Prospective, Multicenter, Clinical and Radiographic Evaluation of a Biointegrative, Fiber-Reinforced Implant for Proximal Interphalangeal Joint ArthrodesisLuke D. Cicchinelli, DPM, Jurij Štalc, MD, Martinus Richter, MD, PhD, Stuart Miller, MD Twenty-five patients underwent PIPJ fusions with OSSIOfiber HammertoeContinue reading “First-in-Human, Prospective, Multicenter Clinical Study”
Co-Principal Investigator’s Overview of First-in-Human Clinical Study
The first-in-human clinical study for OSSIOfiber Intelligent Bone Regeneration Technology was a multicenter, prospective, single-arm study designed to primarily focus on proving the safety and efficacy of the material technology for hammertoe correction arthrodesis. At 6 months, the study’s results were published in Foot & Ankle Orthopaedic Journal. Hear co-principal investigator, Luke Cicchinelli, DPM, speakContinue reading “Co-Principal Investigator’s Overview of First-in-Human Clinical Study”